Abstract
Introduction: Sustained BTK inhibition alone is insufficient to achieve deep response in CLL. Fludarabine can effectively debulk leukemic cells, and target dysfunctional T cells. To determine if the addition of fludarabine can augment depth of response and favorably modulate the T-cell compartment, we conducted a pilot phase II trial using ibrutinib and short-course fludarabine in patients with treatment-naïve (TN) CLL.
Methods and Patients: This prospective, open-label, investigator-initiated phase II trial investigated the efficacy and safety of ibrutinib 420mg once daily, and two cycles of fludarabine (25mg/m2 IV on day 1-5 of cycle 3 and 4, 20% dose reduced if CrCl <70mL/min/1.73m2) (NCT02514083). Eligible patients had TN CLL, irrespective of high-risk genetics, and adequate organ function. Treatment response was assessed based on 2008 iwCLL criteria with 2012 clarifications for treatment-related lymphocytosis. Primary efficacy endpoint was the CR rate after cycle 6. The study was conducted in a Simon's minimax two-stage design; if ³1 CRs occurs in 13 patients enrolled in the first stage, an additional 14 patients will be enrolled in the second stage.
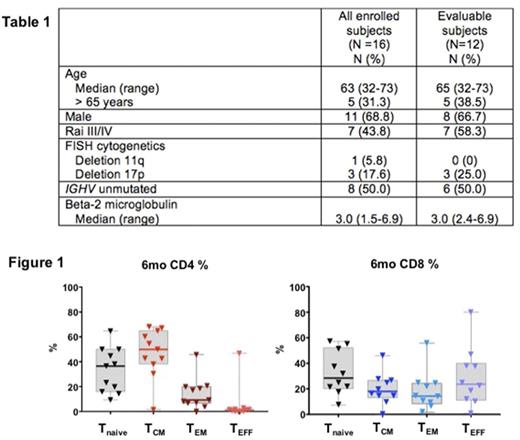
Result: 16 patients were enrolled between December 2015 and June 2017. Median age was 63 years. Baseline characteristics are summarized in Table 1. Four patients on study did not reach the primary endpoint at the time of data analyses. Of 12 patients who reached the primary endpoint, 10 (83%) patients completed planned 6 cycles of ibrutinib and two cycles of fludarabine. One patient stopped treatment after cycle 3 due to grade 3 transaminase elevation. One patient did not complete the second cycle of fludarabine due to intercurrent sepsis, and continued treatment with single-agent ibrutinib thereafter. Dose delays of fludarabine occurred in six (50%) patients due to thrombocytopenia (n=2), infection (n=2), gallstone pancreatitis (n=1), and transaminase elevation (n=1). After 6 cycles, overall response rate was 100%, including CR in 3 (25%) of 12, partial response (PR) in 8 (67%), and PR with lymphocytosis in one (8%). Eight (67%) patients developed redistribution lymphocytosis of 8 weeks median duration (range 4-41). Lymphocytosis resolved in all patients during follow-up. We explored changes in T cells before and after therapy using 8-color peripheral blood flow cytometry. At pre-treatment, patients had increased numbers of T cells that were predominantly CD4+ (median number of CD3+ T: 3,358 cells/uL; median CD4/8 ratio: 2.2). After 6 cycles, CD3+ T cells were in the normal ranges (median 450 cells/uL) with unchanged CD4/8 ratio (median 2.1). We investigated four functional subsets of residual T cells after 6 cycles of therapy based on surface expression of CD62L and CD45RA (Figure 1); central memory T (TCM; CD62LHIGH CD45RALOW), effector T (TEFF; CD62LLOW CD45RAHIGH), effector memory T(TEM; CD62LOW CD45RALOW), and naïve T cells (Tnaive; CD62HIGH CD45RAHIGH). Among CD4+ T cells, TCM was the most abundant subset (50%), followed by Tnaive (37%) and TEM (9%). The CD8+ T cells predominantly consisted of TEFF (24%) and Tnaive (28%), with a small memory population (12% TCM, 15% TEM). Next, we analyzed PD-1 expression in residual subsets of T cells. PD-1 expression was detected in CD4+ CD45RALOW (43% of CD4+ T) and CD8+ CD45RAHIGH populations (26% CD8+ T), indicating CD4+ TCM and CD8+ TEFF were two key T-cell subsets operating immune checkpoint in CLL.
Conclusion: Combination of ibrutinib and short-course fludarabine demonstrated an acceptable tolerability profile. Compared to the reported experience with single-agent ibrutinib, addition of only two cycles of fludarabine to ibrutinib markedly shortend the duration of lymphocytosis and increased the depth of response, with CRs in 25% of patients after 6 cycles. After 6 cycles, CD4 and CD8 T cell numbers were within the normal range. This study has met the predefined efficacy endpoint in the first stage, and is open for accrual to the second stage. A follow-on study for high-risk or relapsed/refractory CLL adds pembrolizumab to the regimen (NCT03204188).
Wiestner: Pharmacyclics: Research Funding; Acerta Pharma: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal